What is Back-scatter Flow Cytometry?
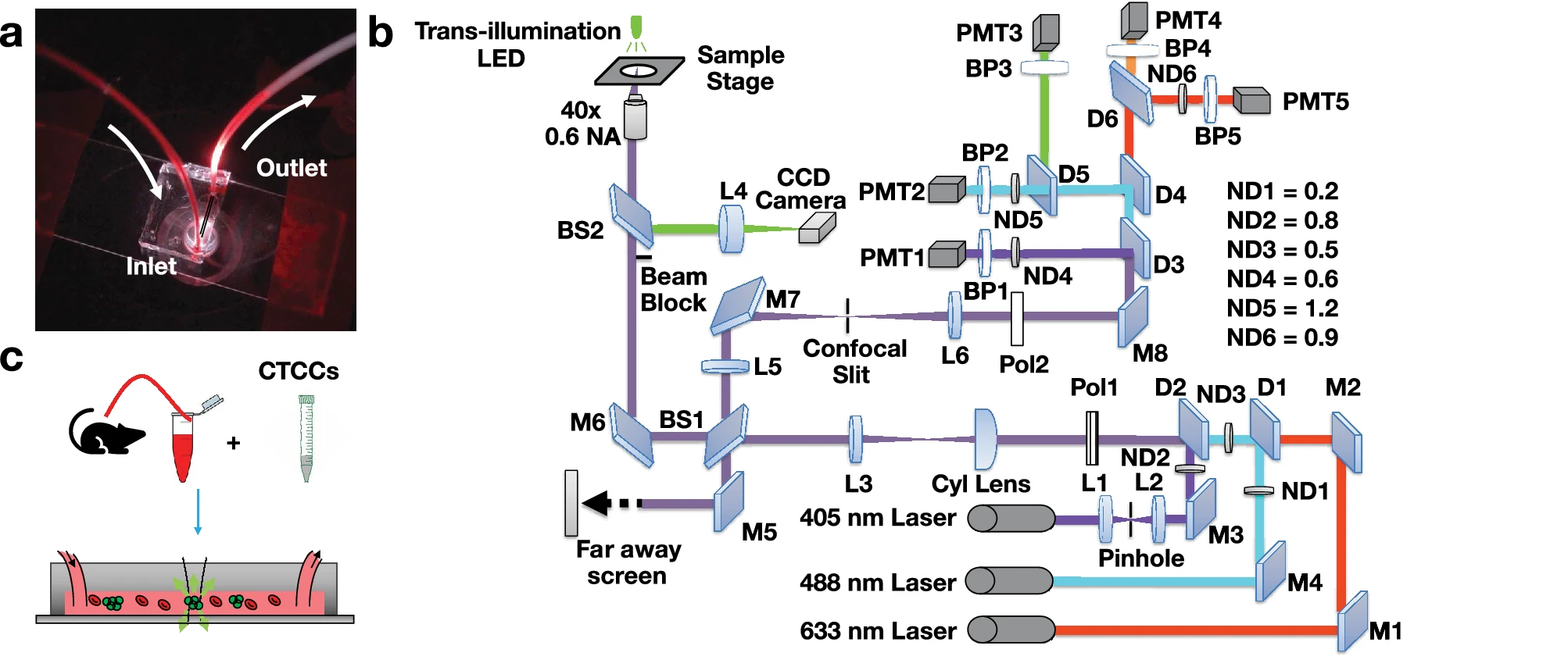
Flow Cytometry is a light-based interrogation method to analyze rare small cellular events in liquid samples. Liquid is pulled into a small channel, often only wide enough for a single cell to pass through. A laser beam is focused through the tube at a orthogonally. As cells cross the laser beam, light scattering in the foward and side directions are collected by specialized detectors called photomultiplier tubes (PMTs). PMTs are specilized low-light detectors that can convert photons into electrical signals that can be registered on a computer. In addition to collecting scattered light signals, specialized markers are used to tag various cellular events in order to distinguish various cell types. These tags are known as fluorescence stains which are attached to specific cells using targetted proteins. Fluorescence is a chemical process under which light is absorbed by the protein marker and then emitted back out as a red-shifted color. Specilized filters can be set up to specifically detect these colors in order to distinguish different cells based on their "glow". Currently, flow cytometry is limited to in vitro use, this means samples must be collected from a patient or animal and then processed outside of the body. Novak et al. (2004) and Georgakoudi et al. (2004) first explored the principles of flow cytometry for in vivo detection of rare circulating tumor cell clusters (CTCCs)5,6.
Back scatter flow cytometry (BSFC) is based on the initial innovations by Novak et al. (2004) and Georgakoudi et al. (2004). Unlike conventional flow cytometry, BSFC collects light scattered back in the same direction as the illumination beam. We utilize three wavelengths - a 405 nm, a 488 nm, and a 633 nm laser - to irradiate our sample. Angled illumination is used in order to block light reflected from the glass slide (Figure 1b). A confocal slit is placed to block out of focus light from reaching the detectors. In addition to collecting scattered light signal, we also collect fluorescence signals from a green fluorescence tag and natural red fluorescence from the cells. The green tag is a useful ground truth label for CTCC identification. A series of specialized mirrors are used to isolate signals from different sources. For greater detail, see Vora et al. (2022). To learn more about IVFC and our BSFC system, click below.
Application of Machine Learning to BSFC Data
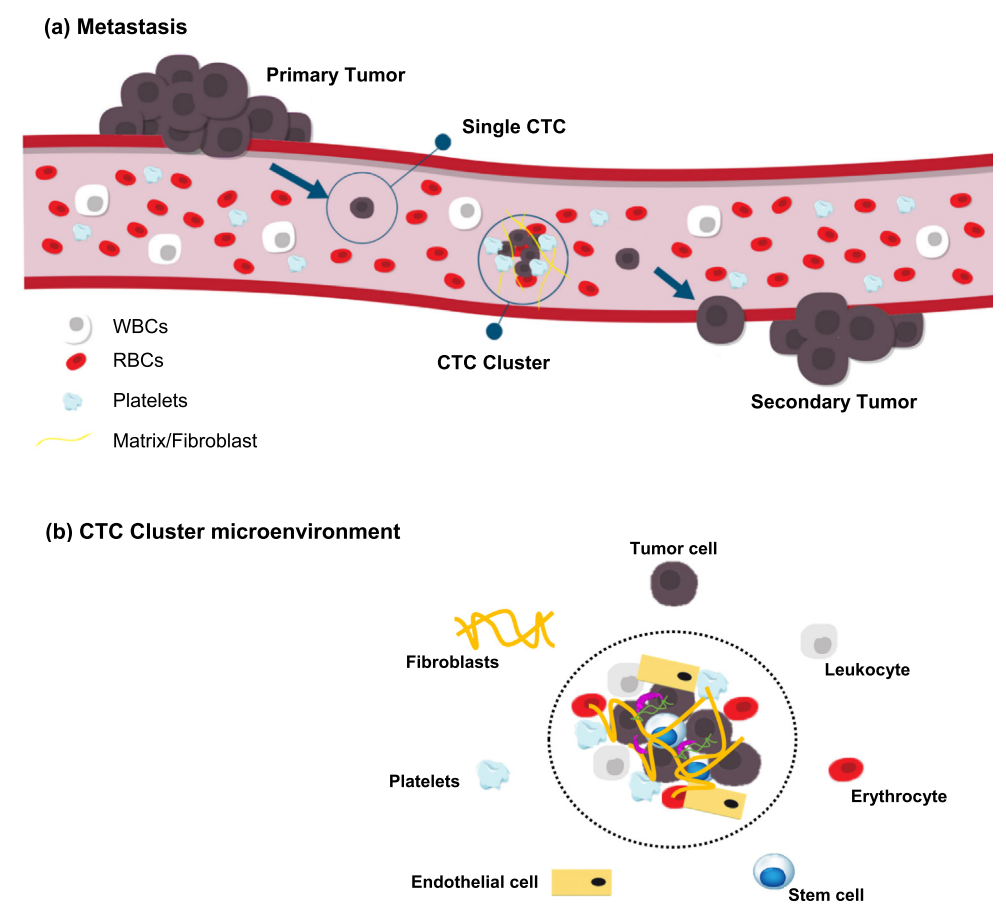
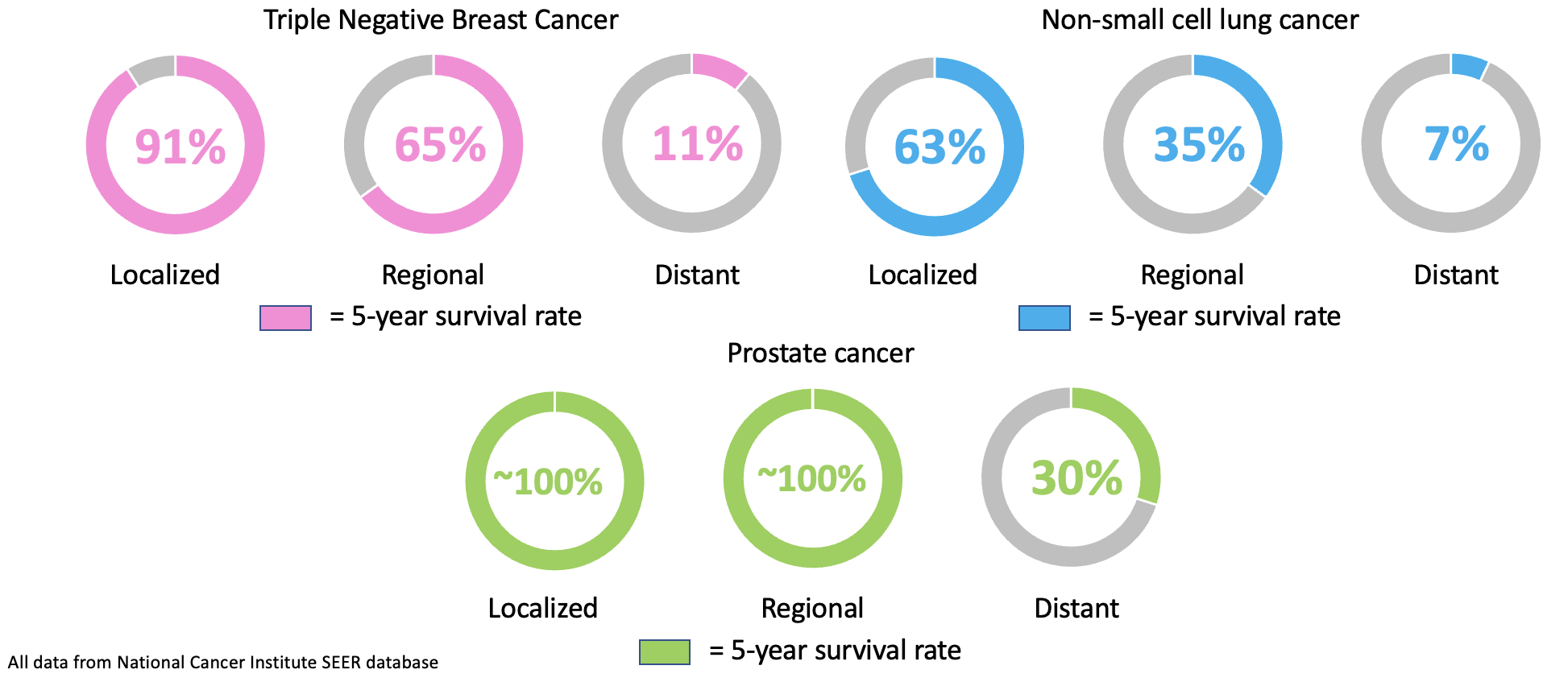
As a tumor grows, individual cells from the tumor will shed from the primary site into the bloodstream4. These individual tumor cells are referred to as circulating tumor cells (CTCs). CTCs occur at a rate of 1-75 CTCs per 7.5 mL of blood while CTCCs occur at an even lower rate of less than 4 CTCCs per 7.5 mL of blood in metastatic cancer patients4. While rare, CTCCs are 23-50 times more likely to lead to metastatic growth compared to individual CTCs1. In vitro detection methods have been widely used to detect CTCCs but can over or under estimate the presence of CTCCs leading to poor correlation with prognosis1. In vivo detection could improve correlation with prognosis with continous monitoring of CTCCs in the blood of patients however, current in vivo flow cytometry (IVFC) is limited by the need for staining of cancer cells or limited application to melanoma (skin cancer)1. We propose a new method of label-free detection of CTCCs in whole blood using light scattering. Using a focused illumination slit, we can estimate the size of cellular events as they cross a 5 μm illuimation slit. Larger cell clusters will feature broader peaks in the collected time-series data which single cell events will feature narrow peak widths. Using a threshold, we can seperate CTCC events from non-CTCC (NC) events, however, while efficient, blood scatter and immune cell response leads to poor signal-to-noise ratio (SNR) and detection of a larger number of false positive events. For successful use of IVFC, it is not only important to be sensitive to CTCC presence but also to be precise in our detection. As such, we decided to explore machine learning techniques for time-series classification of CTCCs in low-SNR scattering datasets.